As researchers learn more and more about our intestinal bacteria—also called the gut microbiome—we're finding out that these microbes aren't just influencing our health and wellness, they're a useful tool for improving it, too.
The bacteria in our intestinal tract must properly process the food we take in to ensure our bodies pull nutrients from what we eat. Beyond processing food, gut bacteria perform functions essential to health, such as vitamin synthesis, immune functions, and defense against infections. Healthy adults have a mixture of different bacteria in their gut.
Research into the effect of diets on the gut microbiome, published in the latest edition of Cell Host & Microbe and led by Jeffrey Gordon from Washington University School of Medicine in St. Louis, has shown that the types of bacteria we have in our gut can be determined by the type of diet we eat. The even better news is that we may be able to acquire good gut bacteria from our housemates who don't eat as much junk food as we do.
Your Bacterial 'Aura'
Factors that determine the composition of our gut bacteria include diet, genetics, antibiotic exposure, and surprisingly, who we live closely with.
According to research led by James Meadow at the University of Oregon in 2015 (he now is Lead Data Scientist at Phylagen), people have a whole host of bacteria that are uniquely associated with their own body. Each of us has a cloud of microbes around us and constantly sheds them into the environment.
We leave these "bacterial signatures" inside buildings, in dust, and on aerosols that come from our bodies. The bacteria that is associated with each of us spreads out into the environment as we touch and breathe on indoor surfaces.
Those bacterial sheddings can be picked up by other people and become part of their bacterial signature. In fact, previous studies have shown that people who live together tend to share gut microbes with each other. Both absorbing bacteria from the environment and our diet itself can modify the bacterial community in our intestines.
Past research has also shown that people whose diets were lower in fruits and vegetables had fewer different types of bacteria in their gut than people who ate more fruits and vegetables. A less diverse gut microbiome is less healthy.
The most recent research about how diet influences gut microbes, by Gordon and team, carefully examined the microorganisms in the guts of people who ate two different types of diets.
One group of 198 people ate a typical American diet (AMER) of whatever they wanted, while the other group of 34 people were on a calorie-restricted diet (CRON) that ate about 50% fewer calories than the usual American diet and had done so for at least two years. The CRON group of people stuck to a plant-based diet and avoided processed foods, refined foods, saturated fatty acids, and carbohydrates known to raise blood sugar quickly—called high glycemic carbohydrates—and exceeded the recommended daily intake of all essential nutrients.
The research team analyzed the bacteria in the feces in the people in each group. They found that people eating the calorie-restricted diet had more types of gut bacteria, and that their gut bacteria was more diverse. More diverse bacteria most likely means that the people who ate the CRON diets could more effectively process their food for energy, nutrition, metabolism, and immune functions. Overall, they had a healthier gut microbiome than the AMER diet eaters.
You Are What You Eat—Or Rather, What Your Gut Bacteria Eat
To investigate how having fewer kinds of gut bacteria might influence weight loss, Gordon and his team had the study participants carefully log what they ate. The food journals helped the researchers to create a diet that closely approximated the calories, fats, and carbohydrates the study participants consumed in either the AMER diet or the CRON diet group.
Then the researchers performed an experiment using four groups of mice.
Into half the mice they implanted the fecal bacteria from humans on the AMER diet. The other half got fecal bacteria from humans on the CRON diet. Feces was deposited directly into the stomach of the mice through a tube put down their throat. The mice were germ-free, meaning they had no gut bacteria of their own. That way, the researchers knew that the gut bacteria in the mice came from the human fecal implants.
To test if it was the calorie reduction or the types of food in the diet that changed the microbiome, they fed half of the mice in one group all they wanted of a diet similar in fats, proteins, sugars, and calories to the AMER diet and the other half got the same food, but could only eat 50% of the daily calories of the typical mouse.
Half of the mice in the other group ate all they wanted of the CRON diet or were fed 50% of it.
The investigators also housed the mice in groups so that some mice who had received feces from humans who ate the AMER diet lived with mice who had received feces from humans who ate the CRON diet. This allowed bacteria to be passed back and forth between mice.
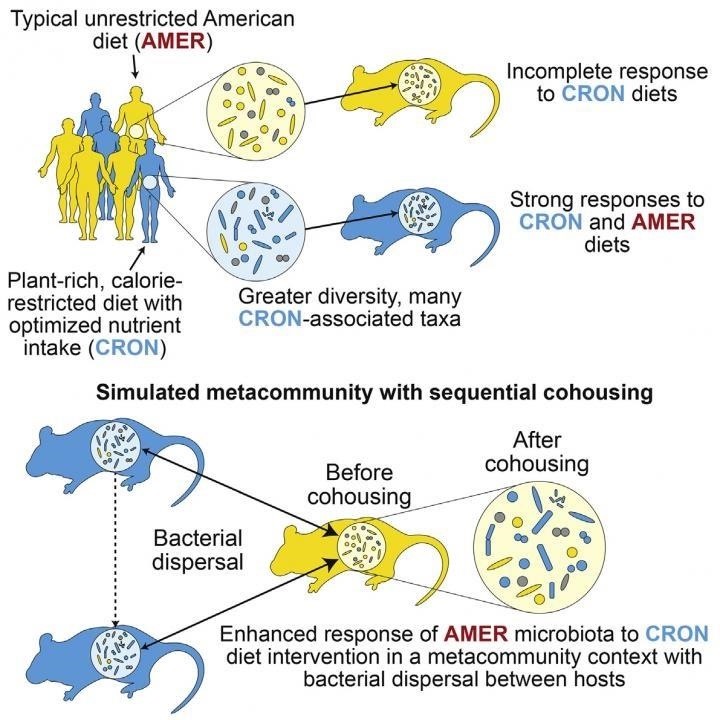
The researchers saw that mice body weights were significantly affected by diet, but not impacted as much by the dietary patterns of the fecal donors.
Mice who ate as much of the AMER diet as they wanted gained about 23% of their starting body weight. When they ate a calorie-restricted AMER diet, they lost about 14.6% of their weight. Mice who ate as much of the CRON diet as they wanted lost 1% of their starting body weight and lost 18.4% when they ate the half as many calories.
The interesting finding here was that the AMER diet led to a big weight gain, but if mice were restricted to half the calories in the AMER diet, they did lose weight. All the mice on the CRON diet lost weight.
The researchers also found that when mice were housed with mice who had different gut bacteria, the cage-mates acquired some of the gut bacteria from the other mice they lived with. For example, when mice with gut bacteria from humans eating the AMER diet were housed with mice colonized with fecal bacteria from CRON-diet eating humans, more diverse and healthier types of bacteria showed up in the AMER mice.
The researchers were able to show that the source of the new bacteria in the AMER-fed mice was the gut of the CRON-fed mice. They had found that good bacteria could colonize the gut of mice who had less diverse bacteria from eating the less nutritional and higher calorie AMER diet—and that this could happen simply by having the mice live together.
What does this mean for human diets and gut bacteria? In a press release from Cell Press, Gordon said, "If we are to prescribe a diet to improve someone's health, it's important that we understand what microbes help control those beneficial effects."
"We hope that microbes identified using approaches such as those described in this study may one day be used as next-generation probiotics," Gordon concluded.
Just updated your iPhone? You'll find new emoji, enhanced security, podcast transcripts, Apple Cash virtual numbers, and other useful features. There are even new additions hidden within Safari. Find out what's new and changed on your iPhone with the iOS 17.4 update.





















Be the First to Comment
Share Your Thoughts